Science Photo Captions
Cover
This image shows a retinal ganglion cell that was biolistically labeled in an adult mouse. Cytosolic expression of fluorescent protein tdTomato (blue) reveals cellular morphology, while coexpression of YFP-tagged PSD95 (yellow) labels excitatory postsynaptic sites within the same neuron. The left portion of the image represents the rendered volume of fluorescent signals expressed by the cell, gradually blended with its digitized representation of the dendritic arbor’s skeleton (blue lines) and synaptic loci (pink spheres).
Courtesy with permission: Yvonne Ou, Rebecca E. Jo, Erik M. Ullian, Rachel O.L. Wong and Luca Della Santina, 2016, Journal of Neuroscience, 36 (35) 9240–9252
Contents
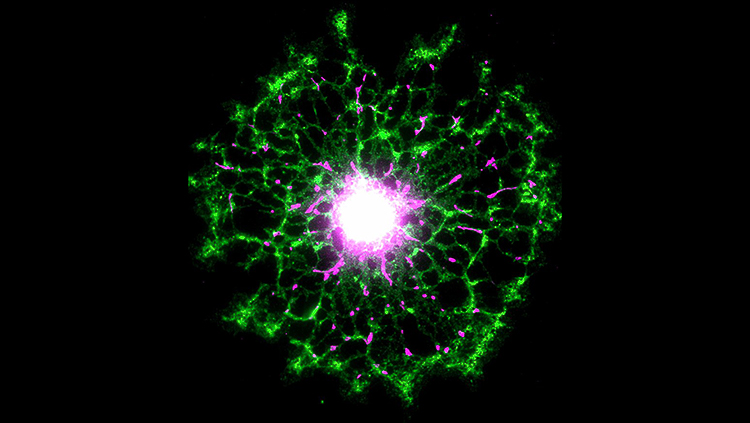
This image shows cone photoreceptors directly contacting microglia within the outer plexiform layer of the human retina. The tissue was immunolabeled with antibodies against calbindin (green) and peanut agglutinin (blue), and microglia were labeled with monocyte marker ionized calcium-binding adapter molecule 1 (red). Microglia, photoreceptor interaction plays an important role in postnatal photoreceptor maturation, with loss of fractalkine-Cx3cr1 signaling leading to an altered distribution of cilium proteins, failure of outer segment elongation, and cone photoreceptor loss.
Courtesy with permission: Andrew I. Jobling, Michelle Waugh, Kirstan A. Vessey, Joanna A. Phipps, Lidia Trogrlic, Una Greferath, Samuel A. Mills, Zhi L. Tan, Michelle M. Ward and Erica L. Fletcher, 2018, Journal of Neuroscience 38 (20) 4708–-4723
Pages viii/01
This image shows specialized functional domains along myelinated axons in mouse sciatic nerve. Immunostaining for neurofascin (blue) is present in the axolemma at nodes of Ranvier (strong staining of NF186) and in paranodal glia (weak staining of NF155). Immunolabeling of contactin-associated protein (green) reveals paranodes, and immuolabeling of voltage-gated K+ channels (red) shows juxtaparanodes.
Courtesy with permission: Keiichiro Susuki, Daniel R. Zollinger, Kae-Jiun Chang, Chuansheng Zhang, Claire Yu-Mei Huang, Chang-Ru Tsai, Mauricio R. Galiano, Yanhong Liu, Savannah D. Benusa, Leonid M. Yermakov, Ryan B. Griggs, Jeffrey L. Dupree and Matthew N. Rasband, 2018, Journal of Neuroscience, 38 (27) 6063–6075
Pages 04/05
This image shows mature cochlear heminodes beneath hair cells and nodes of Ranvier within osseous spiral lamina in adult mouse auditory
nerve. The nodes and their flanking paranodes were immunolabeled for neuronal cell adhesion molecule (NrCAM, green) and contactin 1 (Cntn1, red), respectively. Myelin of the auditory nerve (following the heminodes) was detected by immunolabeling for myelin basic protein (MBP, blue; nuclei were counterstained with DAPI also in blue). The integrity of myelin and nodal structures in the cochlea is needed for fast transfer of sound information from the hair cells to the brain.
Courtesy with permission: Clarisse H. Panganiban, Jeremy L. Barth, Lama Darbelli, Yazhi Xing, Jianning Zhang, Hui Li, Kenyaria V. Noble, Ting Liu, LaShardai N. Brown, Bradley A. Schulte, Stéphane Richard and Hainan Lang, 2018, Journal of Neuroscience, 38 (10) 2551–2568
Page 09
This image shows a cross section of a day 28 human forebrain organoid, showing FOXG1-expressing neural precursors (Red), surrounding a ventricle-like structure outlined by N-Cadherin staining (Green). DAPI staining is blue.
Courtesy with permission: Ai Tian, Julien Muffat and Yun Li, 2020, Journal of Neuroscience, 40 (6) 1186–1193
Page 14/15
This live Airyscan image shows a zebrafish neuromast with hair cells expressing the red calcium indicator RGECO1 (magenta) and the innervating afferent process expressing GFP (neurod: EGFP, green).
Courtesy with permission: Lavinia Sheets, Xinyi J. He, Jennifer Olt, Mary Schreck, Ronald S. Petralia, Ya-Xian Wang, Qiuxiang Zhang, Alisha Beirl, Teresa Nicolson, Walter Marcotti, Josef G. Trapani and Katie S. Kindt, 2017, Journal of Neuroscience, 37 (26) 6299-6313
Page 17 (top image)
This image shows mitochondria (magenta) in the processes of primary oligodendrocytes expressing myelin basic protein (green). The oligodendrocytes were purified via magnetic activated cell separation and cultured for 10 days in vitro.
Courtesy with permission: Kelly A. Chamberlain, Kristen S. Chapey, Sonia E. Nanescu and Jeffrey K. Huang, 2017, Journal of Neuroscience, 37 (6) 1479–1492
Page 17 (middle image)
These vasoactive intestinal peptide (VIP)-expressing interneurons in mouse somatosensory cortex were targeted with a modified rabies virus system to label their brain-wide monosynaptic inputs. Many VIP-expressing interneurons exhibit a striking bipolar morphology, with primary neurites that run perpendicular to the cortical surface.
Courtesy with permission: Nicholas R. Wall, Mauricio De La Parra, Jordan M. Sorokin, Hiroki Taniguchi, Z. Josh Huang and Edward M. Callaway, 2016, Journal of Neuroscience 36 (14) 4000–4009
Page 17 (bottom image)
Abundant α-synuclein inclusions (green) localize throughout axons (magenta).
Courtesy with permission: Laura A. Volpicelli-Daley, Hisham Abdelmotilib, Zhiyong Liu, Lindsay Stoyka, João Paulo Lima Daher, Austen J. Milnerwood, Vivek K. Unni, Warren D. Hirst, Zhenyu Yue, Hien T. Zhao, Kyle Fraser, Richard E. Kennedy and Andrew B. West, 2016, Journal of Neuroscience, 36 (28) 7415–7427
Page 19
This image acquired with super resolution STED microscopy shows a fixed cortical axonal growth cone stained for F-actin (magenta) in the growth cone periphery and microtubules (cyan) in the center. The entry of single microtubules into filopodia and extension along actin filament bundles is regulated by the microtubule associated protein tau.
Courtesy with permission: Sayantanee Biswas and Katherine Kalil, 2018, Journal of Neuroscience, 38 (2) 291–307
Pages 20/21
This image shows a cortical neuron from an embryonic day 14 wild-type mouse grown in culture for 7 days. The cell was immunostained with antibodies against phosphorylated Src/Fyn (pY527, green), Src (red), and the microtubule-associated protein MAP2 (blue) antibodies. The pY527 signal is localized in dendritic growth cones. Sema3A stimulation decreases the pY527 signal in wild-type neurons, but not in those lacking the protein tyrosine phosphatase Ptpδ.
Courtesy with permission: Fumio Nakamura, Takako Okada, Maria Shishikura, Noriko Uetani, Masahiko Taniguchi, Takeshi Yagi, Yoichiro Iwakura, Toshio Ohshima, Yoshio Goshima and Stephen M. Strittmatter, 2017, Journal of Neuroscience, 37 (30) 7125–7139
Page 27
This watercolor, inspired by the drawings of Ramón y Cajal, shows an oligodendrocyte ensheathing two axons, accompanied by illustrations of the different maturation stages through which the oligodendrocyte progenitor cells pass before becoming mature, myelinating oligodendrocytes. The GTPases R-Ras1 and R-Ras2 are essential regulators of oligodendrocyte development and myelination. Drawing by Daniel Belchi.
Courtesy with permission: Miriam Sanz-Rodriguez, Agnès Gruart, Juan Escudero-Ramirez, Fernando de Castro, José María Delgado-García, Francisco Wandosell and Beatriz Cubelos, 2018, Journal of Neuroscience, 38 (22) 5096–5110
Page 32
This image shows a network of cultured cortical neurons that developed with pharmacologically stimulated Protein Kinase C activity and was stained with antibodies against MAP2 (dendrites and somata; green) and neurofilament (axons; red). Cover art by Samora Okujeni.
Courtesy with permission: Samora Okujeni, Steffen Kandler and Ulrich Egert, 2017, Journal of Neuroscience, 37 (14) 3972–3987
Pages 38/39
This image shows the expression of connexin 43 (Cx43, green) in the ependyma of the spinal cord of a neonatal mouse. Nuclei are stained with DAPI (blue). Communication among ependymal cells via gap junctions decreases in adulthood when the ependymal stem cell niche becomes quiescent but is restored after spinal cord injury suggesting a role of connexin signaling in the resumption of proliferation.
Courtesy with permission: Gabriela Fabbiani, Cecilia Reali, Adrián Valentín-Kahan, María Inés Rehermann, Jimena Fagetti, María Victoria Falco and Raúl E. Russo, 2020, Journal of Neuroscience, 40 (11) 2246–2258
Pages 44/45
This image shows neuronal precursor cells obtained from induced pluripotent stem cells stained for neuronal markers Nestin (green) and Sox2 (red), as well as nuclear marker DAPI (blue).
Courtesy with permission: Caterina Montani, Mariana Ramos-Brossier, Luisa Ponzoni, Laura Gritti, Andrzej W. Cwetsch, Daniela Braida, Yoann Saillour, Benedetta Terragni, Massimo Mantegazza, Mariaelvina Sala, Chiara Verpelli, Pierre Billuart and Carlo Sala, 2017, Journal of Neuroscience, 37 (28) 6606–6627
Page 47
The image shows co-injection of two tracers into the Giant Fiber Interneuron (GFI) of the Drosophila thoracic ganglia. TRITC (red) labels the injected neuron while the smaller neurobiotin (yellow) passes through gap junctions to reveal the extensive connectivity of the neural circuit. This brain lacks Fragile X Mental Retardation Protein (FMRP) which results in a selective increase in neurobiotin uptake via a mechanism unrelated to gap junctions.
Courtesy with permission: Tyler Kennedy and Kendal Broadie, 2017, Journal of Neuroscience 37 (41) 9844-9858
Page 49/50
Neuromuscular junction of epitrochleoanconeus (ETA) muscle. ETA muscles of P30 C57/BL6 mouse were stained with neurofilament and synapsin antibodies to visualize motor axons (green) and CF568 α-BTX to visualize AChR clusters (red).
Courtesy with permission: Kai Zhao, Chengyong Shen, Yisheng Lu, Zhihui Huang, Lei Li, Christopher D. Rand, Jinxiu Pan, Xiang-Dong Sun, Zhibing Tan, Hongsheng Wang, Guanglin Xing, Yu Cao, Guoqing Hu, Jiliang Zhou, Wen-Cheng Xiong and Lin Mei, 2017, Journal of Neuroscience, 37 (13) 3465–3477
Page 56
This image shows a cultured hippocampal neuron that was nimmunolabeled for the vesicle SNARE protein synaptobrevin 2. The original image has been artificially colored with a gradient map for artistic effect. The transmembrane domain of synaptobrevin 2 influences the flow of neurotransmitter through synaptic fusion pores.
Courtesy with permission: Chung-Wei Chiang, Che-Wei Chang and Meyer B. Jackson, 2018, Journal of Neuroscience, 38 (32) 7179–7191
Page 59
Confocal image of flat-mounted adult mouse retina showing Iba1-positive ramified microglia (red) of naïve retina. Co-immunostaining for βIII-tubulin (cyan) was used to detect retinal ganglion cells and their axons in the ganglion cell layer.
Courtesy with permission: Alexander M. Hilla, Heike Diekmann and Dietmar Fischer, 2017, Journal of Neuroscience, 37 (25) 6113-6124
Page 60
This image shows expression of an optimized hybrid voltage sensor (hVOS) probe in the dentate gyrus of an Ai35 hVOS:: FOS mouse, viewed with 2-photon microscopy. Neurons expressing the voltage probe were activated by exposing the mouse to a novel environment. The hVOS probe enabled fluorescence imaging of voltage changes in these neurons.
Courtesy with permission: Peter O. Bayguinov, Yihe Ma, Yu Gao, Xinyu Zhao and Meyer B. Jackson, 2017, Journal of Neuroscience, 37 (38) 9305–9319
Pages 62/63
This image shows the results of a clustering model using 20 cortical targets ontology and 29 basal forebrain cell clusters. Each cluster is indicated by a different color. Left is lateral (globus pallidus), right part is medial septum). The brain-model is viewed from an anterior view that is slightly rotated lateralwards. Yellow wireframe: corpus callosum, white: contour of the brain.
Courtesy with permission: Laszlo Záborszky, Peter Gombkoto, Peter Varsanyi, Matthew R. Gielow, Gina Poe, Lorna W. Role, Mala Ananth, Prithviraj Rajebhosale, David A. Talmage, Michael E. Hasselmo, Holger Dannenberg, Victor H. Minces and Andrea A. Chiba, 2018, Journal of Neuroscience, 2018, 38 (44) 9446–9458
Pages 66/67
This image shows that a rainbow enhancer restrictively expressed green fluorescent protein in red, green, and blue (RGB) cones in the zebrafish retina. In zebrafish, RGB cones are structurally similar and unite into mirror-symmetric pentamers (G-R-B-R-G) by adhesion. This structural commonality and unity suggests that a set of genes is commonly and restrictively expressed in RGB cones but not in other cells; rainbow enhancers may represent a cis-regulatory mechanism that underlies such transcriptional regulation to ultimately define the functions of RGB cones, which largely constitute the beginning of the color vision pathway.
Courtesy with permission: Wei Fang, Chuanyu Guo and Xiangyun Wei, 2017, Journal of Neuroscience, 37 (11) 2834–2848
Page 70
A thalamocortical slice from a 4-dayold mouse brain in which neurons in the ventrobasal thalamus express Cre recombinase and tdTomato, allowing visualization of thalamocortical axons (red) innervating the barrel cortex. Layer 6 corticothalamic neurons (green) were labeled by an antibody to the transcription factor TBR1, and all other cell bodies were counterstained with ToPro (blue). The same Cre line was crossed with a channelrhodopsin reporter for optogenetically guided dual recording experiments from connected thalamic and cortical neurons.
Courtesy with permission: Hang Hu and Ariel Agmon, 2016, Journal of Neuroscience, 36 (26) 6906-6916
Pages 78/79
This image shows the mouse adult hippocampus with neurogenesis markers. EYFP (green) is expressed in radial glia-like neural stem cells and their progenies. Adult-born neurons and neural stem cells/neural progenitors are stained with Doublecortin (red) and Sox2 (white), respectively. DAPI labeling is blue.
Courtesy with permission: H. Georg Kuhn, Tomohisa Toda and Fred H. Gage, 2018, Journal of Neuroscience, 38 (49) 10401–10410
Pages 84/85
This confocal image shows a coronal slice of the entire olfactory bulb from a mouse in which EGFP (green) was expressed in calretinin-expressing
(CR+) periglomerular (PG) cells, the most abundant interneurons in the glomerular layer, and a tdTomatoexpressing plasmid (red) had been electroporated into the dorsal subventricular zone at birth to label newborn neurons. Blue DAPI labeling shows nuclei. New work suggests that postnatally generated CR+ PG cells continuously supply the olfactory bulb with a large pool of neurons that have unconventional properties.
Courtesy with permission: Nuria Benito, Elodie Gaborieau, Alvaro Sanz Diez, Seher Kosar, Louis Foucault, Olivier Raineteau and Didier De Saint Jan, 2018, Journal of Neuroscience, 38 (46) 9870–9882
Page 87
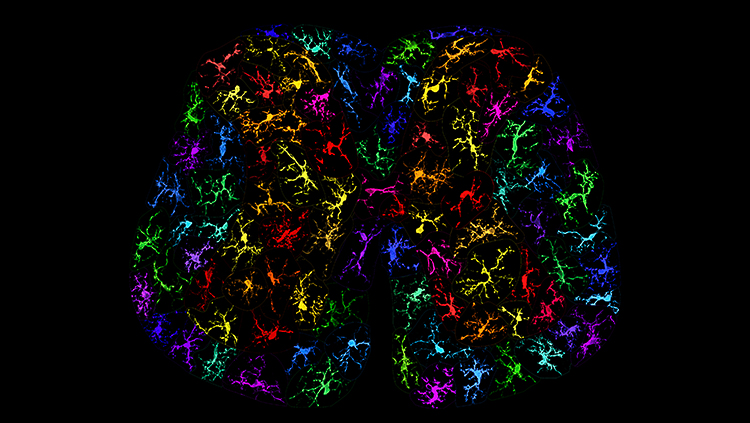
Spinal microglia are critical mediators in the development of opioid tolerance. In this image, a variety of individual Iba1-labelled microglia from rat spinal dorsal horn sections are re-colored and compiled to form a cross-section of the lumbar spinal cord. Each microglia cell is unique and they show a variety of morphologies representative of the dynamic and reactive nature of microglia.
Courtesy with permission: Heather Leduc-Pessah, Nicholas L. Weilinger, Churmy Y. Fan, Nicole E. Burma, Roger J. Thompson and Tuan Trang, 2017, Journal of Neuroscience, 37 (42) 10154–10172
Pages 90/91
This image is an artistic rendering of mouse hippocampus, stained with antibodies against α-synuclein (yellow) and the sphingolipid glucosylceramide (blue). α-Synuclein interacts with select sphingolipids in the context of GBA-associated Parkinson’s disease.
Courtesy with permission: Yumiko V. Taguchi, Jun Liu, Jiapeng Ruan, Joshua Pacheco, Xiaokui Zhang, Justin Abbasi, Joan Keutzer, Pramod K. Mistry and Sreeganga S. Chandra, 2017, Journal of Neuroscience, 37 (40) 9617-9631
Pages 94/95
Unprocessed pro-Neuregulin 1 (type I) accumulates as discrete puncta on the soma and proximal dendrites of cultured hippocampal neurons at contact sites, known as subsurface cisterns, between the somatic plasma membrane and the ER (white). Note that Neuregulin puncta are absent from axons (initial segments labeled with Ankyrin G, green) and more distal dendrites (labeled with MAP2, magenta). In response to NMDAR activity pro-NRG1 is processed and released.
Courtesy with permission: Detlef Vullhorst, Tanveer Ahmad, Irina Karavanova, Carolyn Keating and Andres Buonanno, 2017, Journal of Neuroscience, 37 (21) 5232–5249
Pages 100/101
This image provides a sagittal view of white matter fiber tracts in the human brain obtained using Diffusion Spectral Imaging, a technique explored by the NIH Human Connectome Project and advanced by BRAIN Initiative projects. The work from which the image originated was from the lab of BRAIN-funded investigator, Lawrence Wald, Ph.D. (MGH/Martinos Center for Biomedical Imaging) Setsompop et al., 2013, Neuroimage. The image was provided by the National Institutes of Health, one of the federal agencies supporting the Initiative.
Courtesy with permission: Walter Koroshetz, Joshua Gordon, Amy Adams, Andrea Beckel-Mitchener, James Churchill, Gregory Farber, Michelle Freund, Jim Gnadt, Nina S. Hsu, Nicholas Langhals, Sarah Lisanby, Guoying Liu, Grace C.Y. Peng, Khara Ramos, Michael Steinmetz, Edmund Talley and Samantha White, 2018, Journal of Neuroscience, 38 (29) 6427–6438
Page 103
This image is an artistic rendering of a confocal image depicting parvalbumin-positive inhibitory interneurons (green) intermingled with medium spiny neurons expressing D1 dopamine receptors (pink) in the nucleus accumbens. DAPI labeling is blue. Interneurons are strongly activated by hippocampal input and provide robust feed-forward inhibition to both D1-positive and D1-negative medium spiny neurons.
Courtesy with permission: Samantha L. Scudder, Corey Baimel, Emma E. Macdonald and Adam G. Carter, 2018, Journal of Neuroscience, 38 (42)
9091–9104
Pages 104/105
This image shows immature neurons in layer II of sheep cerebral cortex. Neurons labelled in red express the cytoskeletal protein Doublecortin, typically found in immature or newly-generated cells. The marker of mature neurons HuC/D (green) is present in several nerve cells of the same layer, and shows a faint immunoreactivity only in a subset of Doublecortin-positive cells, thus indicating the existence of different degrees of immaturity. Neurons generated embryonically but remaining immature in adults are particularly abundant in large-brained, long-living mammals such as sheep.
Courtesy with permission: Matteo Piumatti, Ottavia Palazzo, Chiara La Rosa, Paola Crociara, Roberta Parolisi, Federico Luzzati, Frederic Lévy and Luca Bonfanti, 2018, Journal of Neuroscience, 38 (4) 826–842
Page 106 (top image)
This composite image shows channelrhodopsinexpressing axons of the lateral perforant path (green) targeting dendritic segments of granule cells (red) in the outer molecular layer of the mouse hippocampus. Laminar-specific activation showed that newborn cells receive strong preferential input from the lateral perforant path, despite showing similar spine density and dendritic length in the middle (light blue) and outer (light purple) molecular layer, a feature that may support their unique role in pattern separation.
Courtesy with permission: Nicholas I. Woods, Christopher E. Vaaga, Christina Chatzi, Jaimie D. Adelson, Matthew F. Collie, Julia V. Perederiy, Kenneth R. Tovar and Gary L. Westbrook, 2018, Journal of Neuroscience, 38 (26) 5843–5853
Pages 106/107 (bottom image)
This image shows ventral CA1 hippocampal (vCA1) neurons that project to either the mPFC (green) or amygdala alone (red), as well as vCA1 neurons that project to both areas (yellow). The vCA1 neurons were labeled using a dual retrograde viral tracing approach.
Courtesy with permission: Woong Bin Kim and Jun-Hyeong Cho, 2017, Journal of Neuroscience, 37 (19) 4868–4882
Pages 114/115
This image shows the spatial distribution of F-actin in a growth cone, as revealed using structured illumination microscopy (SIM), one type of
superresolution microscopy. Colors indicate the height from the substrate.
Courtesy with permission: Michihiro Igarashi, Motohiro Nozumi, Ling-Gang Wu, Francesca Cella Zanacchi, István Katona, László Barna, Pingyong Xu, Mingshu Zhang, Fudong Xue and Edward Boyden, 2018, Journal of Neuroscience, 38 (44) 9459–9467
Page 118
In the sympathetic trunk of a CNPMyrAkt mouse, an unmyelinated Schwann cell (brown) engulfs one axon (violet) but also collagen fibers from the extracellular matrix (blue, orange). Next to it, long processes from another unmyelinated Schwann cell (yellow) are starting to wrap extracellular matrix.
Courtesy with permission: Enric Domènech-Estévez, Hasna Baloui, Xiaosong Meng, Yanqing Zhang, Katrin Deinhardt, Jeff L. Dupree, Steven Einheber, Roman Chrast and James L. Salzer, 2016, Journal of Neuroscience, 36 (16) 4506–4521
Pages 124/125
This image shows a dopaminergic neuron immunostained for cytosolic tyrosine hydroxylase (red), plasma-membrane-bound dopamine transporter (green), and nuclear DAPI staining (blue). The neuron was generated from BMP5/7-treated human induced pluripotent stem cells. The BMP/SMAD pathway has a critical role in the formation of dopaminergic neurons in vivo and from human stem cells.
Courtesy with permission: Vukasin M. Jovanovic, Ahmad Salti, Hadas Tilleman, Ksenija Zega, Marin M. Jukic, Hongyan Zou, Roland H. Friedel, Nilima Prakash, Sandra Blaess, Frank Edenhofer and Claude Brodski, 2018, Journal of Neuroscience, 38 (7) 1662–1676
Pages 130/131
This image shows human neurons derived from embryonic stem cells (H9), at 21 days after differentiation. Nuclei are stained with DAPI and the neuronal microtubules are stained with an antibody against β-III-Tubulin. These cells were used to test candidate modifiers of α-Synuclein levels.
Courtesy with permission: W.C. Rousseaux, Gabriel E. Vázquez-Vélez, Ismael Al-Ramahi, Hyun-Hwan Jeong, Aleksandar Bajić, Jean-Pierre Revelli, Hui Ye, Emily T. Phan, Jennifer M. Deger, Alma M. Perez, Ji-Yoen Kim, Laura A. Lavery, Qikia Xu, Mamie Z. Li, Hyojin Kang, Jean J. Kim, Joshua M. Shulman, Thomas F. Westbrook, Stephen J. Elledge, Zhandong Liu, Juan Botas and Huda Y. Zoghbi, 2018, Journal of Neuroscience, 38 (43) 9286–9301
Page 133
Longitudinal section of adult rat optic nerve, immunostained for Thr286-phosphorylated CaMKII (pT286; green), myelin basic protein (MPB; magenta), and axonal neurofilaments (SMI-312; blue). pT286 localizes to axons and not to myelin, as SMI-312 and pT286 colocalization generates cyan within profiles circumscribed by MBP. The axon diameters range between small and large. A few large gaps between axon fascicles show some pT286 immunopositivity, but no signal for MBP or SMI-312.
Courtesy with permission: Gloria J. Partida, Anna Fasoli, Alex Fogli Iseppe, Genki Ogata, Jeffrey S. Johnson, Vithya Thambiaiyah, Christopher L. Passaglia and Andrew T. Ishida, 2018, Journal of Neuroscience, 38 (37) 8087–8105
Pages 134/135
Retinal ganglion cell axons from the ventral (green) and dorsal (red) retina are segregated in the developing optic tract of zebrafish embryos. This
sorting is disrupted when the RNA-binding protein Hermes is knocked down.
Courtesy with permission: Hörnberg, Jean-Michel Cioni, William A. Harris and Christine E. Holt, 2016, Journal of Neuroscience, 36 (50) 12697–12706
Pages 138
Immunohistochemical labeling of parvalbuminexpressing inhibitory interneurons (yellow) and cell nuclei (DAPI, purple) in the somatosensory cortex of a postnatal day 15 Dp(16)1Yey/+ mouse (a mouse model of Down syndrome). Photoshop was used to adjust the hue and contrast of the image.
Courtesy with permission: Joseph W. Goodliffe, Jose Luis Olmos-Serrano, Nadine M. Aziz, Jeroen L.A. Pennings, Faycal Guedj, Diana W. Bianchi and Tarik F. Haydar, 2016, Journal of Neuroscience, 36 (10) 2926–2944
Pages 142/143
This image shows two human astrocytes derived from embryonic stem cells stained with F-actin (green). The astrocytes were exposed to Cy-3 labeled alphasynuclein oligomers (red) for 24 hours, washed and cultured for additional three days. During the 24 h of exposure, the astrocytes engulf large amounts of oligomeric alpha-synuclein that are subsequently accumulated in the cells. The stressed astrocytes respond by sending out tunneling nanotubes, enabling intercellular transfer of alphasynuclein.
Courtesy with permission: Jinar Rostami, Staffan Holmqvist, Veronica Lindström, Jessica Sigvardson, Gunilla T Westermark, Martin Ingelsson, Joakim Bergström, Laurent Roybon and Anna Erlandsson, 2017, Journal of Neuroscience, 37 (49) 11835–11853
Pages 144
This image shows the mouse adult hippocampus with neurogenesis markers. EYFP (green) is expressed in radial glia-like neural stem cells and their progenies. Adult-born neurons and neural stem cells/neural progenitors are stained with Doublecortin (red) and Sox2 (white), respectively. DAPI labeling is blue.
Courtesy with permission: H. Georg Kuhn, Tomohisa Toda and Fred H. Gage, 2018, Journal of Neuroscience, 38 (49) 10401–10410
Pages 146/147
This image shows neurons (green) and ephrin-B1 immunoreactivity (red) in mouse hippocampal area CA1 of adult mice that overexpress ephrin-B1 in astrocytes. Cell nuclei are labeled with DAPI (blue).
Courtesy with permission: Jordan Koeppen, Amanda Q. Nguyen, Angeliki M. Nikolakopoulou, Michael Garcia, Sandy Hanna, Simone Woodruff, Zoe Figueroa, Andre Obenaus and Iryna M. Ethell, 2018, Journal of Neuroscience, 38 (25) 5710–5726
Pages 152/153
Reconstruction of a cluster of seven simultaneously recorded neurons in the subthalamic nucleus. Dendrites and somata of each cell are a different color, but axons of all cells are red. New work shows that subthalamic nucleus neurons operate as independent and parallel processing units.
Courtesy with permission: Leon Amadeus Steiner, Federico J. Barreda Tomás, Henrike Planert, Henrik Alle, Imre Vida and Jörg R.P. Geiger, 2019, Journal of Neuroscience, 39 (13) 2470–2481
Related