Inside Neuroscience: The Relationship Between Alzheimer’s Disease and Diabetes
Alzheimer’s disease is the most common form of dementia, affecting over 50 million people worldwide, with rates expected to triple in the next 30 years. The disease is characterized by aggregates of amyloid beta outside neurons and tangles of hyperphosphorylated tau within neurons. More recently, evidence suggests a disturbance in glucose metabolism in the Alzheimer’s brain as well, although whether this is a cause or symptom of the disease remains unclear. Type 2 diabetes — in which blood glucose levels are elevated — increases the risk for Alzheimer’s disease about two-fold.
A better understanding of the relationship between these two diseases may yield novel therapies. At the Neuroscience 2019 press conference “Untangling the Link Between Diabetes and Alzheimer’s Disease,” researchers shared new insights into how glucose metabolism problems and Alzheimer’s pathology could be related, disrupted, and potentially fixed.
“This is a really hot topic right now,” said David Holtzman, professor of neurology at Washington University, scientific director of the Hope Center for Neurological Disorders, and the session’s moderator. “Because the prevalence of Alzheimer’s disease is so high, especially as we get over the age of 70, this has really become a public health crisis.”
Disrupted Insulin Signaling in Alzheimer’s Mice Leads to Memory Impairment
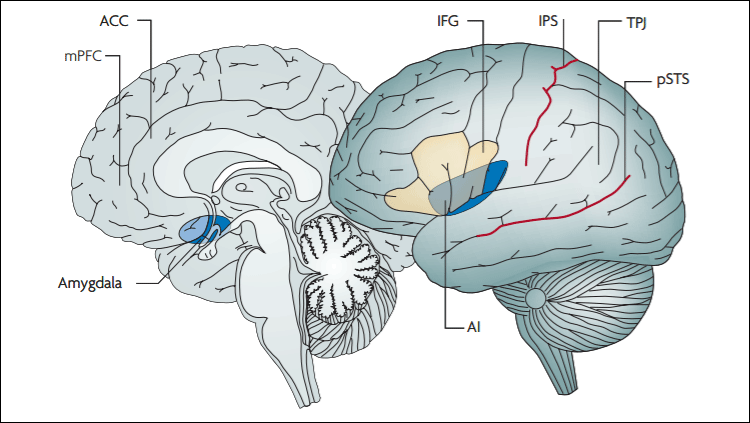
Both Alzheimer’s disease and diabetes involve impaired insulin signaling. The insulin signaling pathway includes the kinase GS3K, which is a major player in hyperphosphorylated tau, a hallmark pathology of Alzheimer’s disease.

Sami Gabbouj, doctoral student, department of neuroscience, University of Eastern Finland.
Because the typical Western diet — high in fat and carbohydrate and low in fiber — is a major risk factor for diabetes, researchers at the University of Eastern Finland wondered if such a diet also affects the development of Alzheimer’s disease. The team found that, in female mice from four different Alzheimer’s models, the typical Western diet induced diabetes-like symptoms, including obesity and impaired glucose tolerance. Compared to the standard rodent diet, the typical Western diet led to decreased activity of an insulin signaling pathway and inhibition of GS3K — which can exacerbate Alzheimer’s pathology — in the hippocampus of female mice. The blunted insulin signaling also correlated with age-related memory impairment on a swim test.
“The typical Western diet appears to disturb insulin signaling in the brain and dysfunctional brain insulin signaling may promote Alzheimer’s disease progression,” said Sami Gabbouj, a doctoral student in the department of neuroscience and presenter of the work.
Glucose Transporter Decreased in Alzheimer’s Mice

Steven Barger, professor of geriatrics, neurobiology and developmental sciences, and internal medicine, University of Arkansas for Medical Sciences.
Alzheimer’s disease patients and diabetics also both have decreased utilization of glucose in the brain. Steven W. Barger, a professor of geriatrics, neurobiology and developmental sciences, and internal medicine at the University of Arkansas for Medical Sciences, and his team found a mouse model of Alzheimer’s disease exhibited elevated levels of glucose in the blood, similar to the diabetes manifest in mice made obese by a typical Western diet. However, while the obese mice had poor responses to insulin, the Alzheimer-like mice did not, suggesting problems with an insulin-independent tissue. Consistent with this, the Alzheimer-like mice also took glucose up into the brain less efficiently (similar to human patients). This was accompanied by a 50% decrease in the insertion of glucose transporter GLUT1 into the plasma membrane of astrocytes. “Glucose is not being efficiently transported into the brain and therefore remains in the blood, and that sort of mimics what you would see with excesses of glucose in diabetes,” said Barger.
Barger and his team also found that adding beta-amyloid directly to astrocytes produced the same deficit in GLUT1 localization, indicating the peptide may be responsible for less glucose entering the brain. These results suggest improving glucose delivery to the brain may be an effective treatment for Alzheimer’s disease. A comprehensive report of these findings was published in October.
Better Glucose Metabolism May Confer Protective Effect of APOE2

Liquin Zhao, associate professor of pharmacology and toxicology, University of Kansas
Unfortunately, nearly all clinical trials for Alzheimer’s disease treatments have failed. “Regrettably the pathology-focused approach has not worked well,” said Liqin Zhao, associate professor of pharmacology and toxicology at the University of Kansas. “Maybe we can also approach from the other end of the spectrum, by figuring out what makes some people resilient.” To do this, Zhao studied apolipoprotein E, a gene with three isoforms in humans: APOE2, APOE3, and APOE4. People with at least one copy of APOE4 have increased risk of Alzheimer’s, while people with APOE2 have decreased risk. (APOE3 is considered neutral.)
Zhao found increased glucose breakdown in cells and mouse brains expressing the APOE2 isoform, compared to those expressing APOE3 and APOE4. This was due to upregulation of hexokinase, an enzyme responsible for the first step of glucose metabolism and correlated with better health of the cells. In APOE4-expressing cells and brains, hexokinase was downregulated, resulting in decreased metabolism of glucose and poorer cell health. Treating APOE4-expressing cells with APOE2 reduced the metabolic deficiency, suggesting a novel therapeutic approach that might have more promise.
Metabolism, Sleep, And Alzheimer’s Disease Feed on Each Other
The metabolism of glucose, amyloid beta, and tau are tightly coupled to the sleep-wake cycle: metabolism, amyloid beta levels, and tau levels increase when we’re awake before falling while we sleep. Sleep deprivation disrupts this cyclical pattern and people with sleep disorders have an increased risk for Alzheimer’s disease. Conversely, Alzheimer’s disease pathology — amyloid beta plaques and neurofibrillary tangles — disrupts sleep further.

Shannon Macauley, assistant professor of gerontology and geriatric medicine, Wake Forest School of Medicine
Shannon L. Macauley, assistant professor of gerontology and geriatric medicine at Wake Forest School of Medicine, wondered if both sleep and Alzheimer’s disease pathology are impacted by changes in blood sugar. Using mice, her team found that both high and low blood sugar — both of which can be observed in Type 2 diabetes — increased neuronal activity and decreased sleep. A mouse model with prominent plaque pathology and one with prominent tangle pathology also displayed disrupted brain metabolism and decreased sleep, suggesting an uncoupling of the relationship between metabolism and sleep.
“As you have these fluctuations in your blood glucose levels that remain unchecked, this can interact with the plaques and tangles within your brain to disrupt sleep,” Macauley said.
Body and Brain
The work highlights how understanding and treating diseases of the brain requires considering the body as well. “What’s happening in our periphery is directly affecting our brain,” said Macauley. “If you have fuel excess or impairments in fuel processing, it’s going to kick off a cascade on multiple levels to ramp up Alzheimer’s pathology.” While the research may lead to new treatments, in the meantime it serves as a reminder of the importance of lifestyle interventions, including diet and sleep.